![]() The most talked-about physics paper last week was probably Negative Absolute Temperature for Motional Degrees of Freedom (that link goes to the paywalled journal; there’s also a free arxiv preprint from which the above figure is taken). It’s a catchy but easily misinterpreted title– Negative absolute temperature! Below Absolute Zero! Thermodynamics is wrong!– that obscures the more subtle points of what’s going on here. So, in the interest of clarity, I’m going to attempt an explanation, over the course of a few posts, but given my schedule these days, that might spread over a couple of weeks. So, first, let’s get the question of what “negative absolute temperature” means out of the way, so if nothing else people are (more) clear on that point.
The most talked-about physics paper last week was probably Negative Absolute Temperature for Motional Degrees of Freedom (that link goes to the paywalled journal; there’s also a free arxiv preprint from which the above figure is taken). It’s a catchy but easily misinterpreted title– Negative absolute temperature! Below Absolute Zero! Thermodynamics is wrong!– that obscures the more subtle points of what’s going on here. So, in the interest of clarity, I’m going to attempt an explanation, over the course of a few posts, but given my schedule these days, that might spread over a couple of weeks. So, first, let’s get the question of what “negative absolute temperature” means out of the way, so if nothing else people are (more) clear on that point.
What do you mean, clear it up? I thought it was obvious. Temperature is a measure of how fast atoms are moving, so negative temperature means they’re moving backwards. Yeah, not so much. Atoms don’t have faces, so “forward” and “backward” don’t have any meaning.
In the atomic model, the temperature of a gas is a measure of the average kinetic energy of the atoms making it up. The kinetic energy depends on the speed of the atoms only (the speed squared, provided they’re not moving too fast), not the direction.
But if that’s the case, how can it possibly be negative? It can’t, which is the reason for the “In the atomic model” at the start of the previous paragraph. The relationship between speed and temperature isn’t an absolute fact, just a convenient way to think about temperature in the simple case where you have a gas of atoms that behave like tiny little classical particles. The reality of temperature is a good deal more complex, with a lot of subtleties. It’s not just about the speed at which things are moving.
Why not? What’s subtle about this? Well, for one thing, it’s a characteristic of a distribution of velocities, not a feature having to do with absolute velocity. A more correct statement would be that the temperature is a measure of the spread in energies of a sample of atoms.
I’m not sure I see the point of that. Well, think about a bottle of gas, at room temperature. The atoms in that gas have some speeds relative to the bottle, right? And you would be inclined to say that the temperature is determined by the average of those speeds, yes?
Right, that’s the whole point. OK, now at room temperature, the atoms in a typical gas are moving at something close to the speed of sound. So, if we put that bottle on a jet airplane also traveling at the speed of sound, we must have increased the temperature, right? After all, according to a physicist on the ground, they’re moving from point A to point B at the speed of sound, and their average speed was the speed of sound, so when you add those together, the gas has gotten much hotter.
Ummm… I don’t think that makes sense. Right. Which is why I say that temperature is a property of the distribution of energies, and in fact has to do with the spread of energies. The atoms in the plane are collectively moving at a high speed relative to the observer on the ground, but the distribution of speeds relative to their container has not changed, so the temperature has not changed.
Okay, then, what’s the right way to think about this? Mathematically, physicists talk about the temperature as a property of a distribution of energies, typically a Maxwell-Boltzmann distribution, which has a complicated mathematical form, but is primarily characterized by one factor, which looks like this:
$latex f(E) \propto e^{-E/k_{B}T} $
The little e is the Euler number, 2,718…, and the big E is the energy of a particular state. T is the temperature of the gas, and $latex k_B $ is Boltzmann’s constant, which just gets the units right. This factor, roughly speaking, tells you the probability of finding an atom moving at a speed that corresponds to a given energy E.
The fact that you have used math here makes me very nervous. Please, explain this in words before I’m overcome with the vapors. It’s not that scary. The important thing to take away is that this is an exponential function. If you double the energy E that you’re looking for, keeping the temperature the same, the probability of finding an atom in the gas with that energy drops off by a factor of $latex e^{-2} \approx 0.14$. If you quadruple the energy, it drops off by $latex e^{-4} \approx 0.018 $.
It’s also exponential in the temperature, but in the other direction. If you double the temperature, the probability of finding an atom at a given energy goes up by a factor of 7.4, and so on.
So, if you want to find a high-energy atom, you need a high temperature? Or you need to be very lucky, yes. For any given temperature, the probability of finding an atom with a given energy decreases exponentially as the energy increases.
This holds for all manner of systems, and it even works when you go to quantum-mechanical treatments, where the atoms can’t have just any old energy, but can only be found in certain discrete energy states. The probability of finding a given state occupied decreases exponentially as you increase the energy of that state, and the rate at which it decreases depends on the temperature.
You can also use this to calculate the probability of finding a given velocity, provided you’re dealing with kinetic energy. In the case of the airplane example above, you do it using the velocity relative to the average velocity of the atoms due to the motion of the plane. That gives you a distribution of velocities relative to the ground that looks just like a Maxwell-Boltzmann distribution shifted over to a higher average. For this reason, you’ll hear physicists use seemingly nonsensical phrases like “cold supersonic atomic beam”– what they mean here is that the atoms in a beam are all headed in a particular direction at some high speed, but that the distribution of speeds around that average speed is very narrow, corresponding to a Maxwell-Boltzmann distribution for a very low temperature.
So what does a negative temperature do? Well, if you look at the equation above, you see that you have a negative sign in the exponent. Which is why the factor gets smaller as you go up in energy. If you make the temperature negative, though, that adds another negative sign to the exponent, and a negative times a negative…
… is positive! So, a negative temperature system is one where the probability of finding atoms in a given energy state increases as you increase the energy? Precisely. It increases exponentially, in fact. The rate of increase still depends on the temperature, but with a twist– the probability increases faster for negative temperatures that are small than for negative temperatures that are large (that is, a temperature of -0.1 K produces many more atoms at a given high energy than a temperature of -10 K).
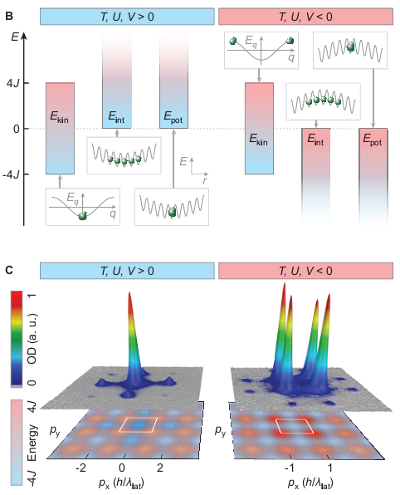
So, this negative temperature stuff is just about probability distributions? Pretty much, yes. Which may not seem all that much more exciting than just making the atoms move backwards, but it’s actually a pretty clever experiment. The tricky thing with making negative-energy systems is that you need to somehow cap the energy, otherwise the Boltzmann factor demands that you have huge numbers of atoms at arbitrarily high energy. Which is what makes this experiment novel– people have demonstrated “negative temperature” systems before for properties like spin, where there’s an intrinsic limit imposed by the system. In this case, they’re dealing with the velocity– that’s what they mean by “motional degrees of freedom”– and have imposed an upper limit on the velocity by putting the atoms in an optical lattice.
How do they do that? That’s an excellent question, but as noted above, I have a million other things to do, so the answer to it is beyond the scope of this blog post. I will try to get to an explanation of how they did this, and how they know that it worked, but it’ll be a little while.
But at least now you have a better idea of what “negative temperature” means, and how it relates to everything else. I hope.
Late addition: Matt Springer at Built On Facts posted nearly simultaneously with me, giving another take on the meaning of negative temperature. With graphs, rather than an equation.
Braun, S., Ronzheimer, J., Schreiber, M., Hodgman, S., Rom, T., Bloch, I., & Schneider, U. (2013). Negative Absolute Temperature for Motional Degrees of Freedom Science, 339 (6115), 52-55 DOI: 10.1126/science.1227831
Thanks for this clarification!
Yes, that is an excellent description. I hadn’t looked into this other than the headlines but I was wondering exactly what ‘negative temperature’ was.
Thanks for the clarification. I had guessed at an interpretation that was similar to what you’ve stated, but less…smart.
Just out of curiosity, does the same explanation work in quantum statistics? In the case of Fermi statistics, negative temperature can just be mapped to a system with anti-particles at positive temperature. I’m wondering if there is some sort of remapping of the relevant degrees of freedom in this system which would then have a positive temperature…
if all articles about the experiment could have this as annex…
may i ask something? i know that they were also blocking tunneling turning the BEC into Mott insulating regime. would be there any differences in quantum tunelling in that negative temperature state? or does the Landau-Zener give any predictions in such states?
I first thought of the ‘negative temperature’ section of the old sci.physics FAQ. See http://math.ucr.edu/home/baez/physics/ParticleAndNuclear/neg_temperature.html for that. It was using a different approach, defining a more fundamental ‘temperature’ in terms of thermodynamics rather than the usual ‘kinetic temperature’ of moving atoms, then creating a quantum spin system which can have either a positive temperature (number of available states increases as energy is input) or a negative temperature (number of available states decreases as energy is input). You still can’t CROSS absolute zero in this system, but as you input energy into the system you can go from zero to positive infinity, wrap to negative infinity, then increase back to zero again.
Excellent; as I said in the other blog, I was skeptical of the popular press renditions of this, and nearest I could guess, it was a way of dealing with negatives in an equation, similar to the way that antimatter serves to address certain theoretical issues in equations.
Since Andrew and I are both guessing in the realm of “analogies with antimatter,” that might be an interesting issue to address in a subsequent posting.
To go a bit further afield: As I understand, one of the theories regarding antimatter is that it can be treated as if it’s matter with the time variable in the equation set to negative, as if it’s matter propagating backward across time. Do similar considerations apply for “negative temperature”? That is, if you have an equation representing change in temperature over time, and change in the Boltzman distribution of atoms over time, can you get to the same result by flipping the time variable to negative, rather than the temperature variable? And does that have any further theoretical implications?
wow great explanation 🙂 makes a lot of sense now, fascinating stuff imo